Cannabidiol: Promise and Pitfalls
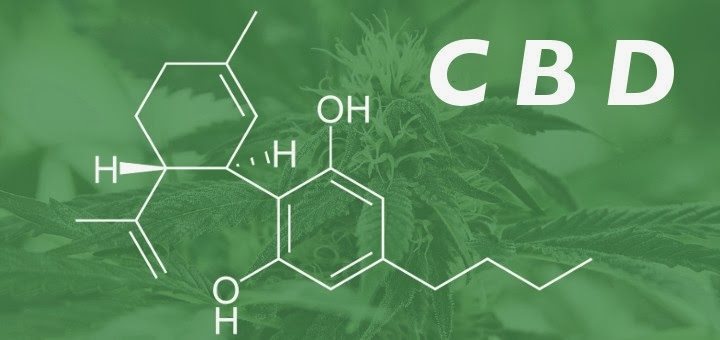

Over the past few years, increasing public and political pressure has supported legalization of medical marijuana. One of the main thrusts in this effort has related to the treatment of refractory epilepsy—especially in children with Dravet syndrome—using cannabidiol (CBD). Despite initiatives in numerous states to at least legalize possession of CBD oil for treating epilepsy, little published evidence is available to prove or disprove the efficacy and safety of CBD in patients with epilepsy. This review highlights some of the basic science theory behind the use of CBD, summarizes published data on clinical use of CBD for epilepsy, and highlights issues related to the use of currently available CBD products.
Cannabidiol is the major nonpsychoactive component of Cannabis sativa. Over the centuries, a number of medicinal preparations derived from C. sativa have been employed for a variety of disorders, including gout, rheumatism, malaria, pain, and fever. These preparations were widely employed as analgesics by Western medical practitioners in the 19th century (1). More recently, there is clinical evidence suggesting efficacy in HIV-associated neuropathic pain, as well as spasms associated with multiple sclerosis (1).
acesse: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4189631/

